主办单位:煤炭科学研究总院有限公司、中国煤炭学会学术期刊工作委员会
Table1
近年CO2加氢制C2+醇的各类Fe基催化剂反应条件及性能
| NO. | Catalyst | t/°C | p/MPa | GHSV /(mL·g−1·h−1) |
CO2 conv./% |
Selectivity/% | C2+OH·STY. /(mg·g−1·h−1) |
Ref. | |||
| CO | CH4 | EtOH | C2+OH | ||||||||
| 1 | CuZnFe0.5K0.15 | 300 | 6.0 | 5000 | 42.3 | 6.9 | − | − | 31.94 | 0.17a | [8] |
| 2 | 0.1Pd/Fe3O4 | 300 | 0.1 | 60000 | 0.3 | − | 0 | 97.5 | 97.5 | 413b | [9] |
| 3 | 4.6K-CMZF | 320 | 5.0 | 6000 | 30.4 | − | − | − | 22.8 | 69.6 | [10] |
| 4 | 3%Cs-C0.8F0.1Z1.0 | 330 | 5.0 | 4500 | 36.6 | 20.6 | ~10 | − | 19.8 | 73.4 | [11] |
| 5 | 2% Na-Fe@C/5% KCuZnAl | 320 | 5.0 | 4500 | 39.2 | 9.4 | ~6 | 35 | 35 | − | [12] |
| 6 | K-CMZF/CZA | 320 | 5.0 | 6000 | 42.3 | 13.8 | 10.1 | 14.7 | 17.4 | 106.5 | [13] |
| 7 | K-0.82-FeIn/Ce-ZrO2-900 | 300 | 10.0 | 4500 | 29.6 | 13.4 | 20.1 | − | 28.7 | − | [14] |
| 8 | FeNaS-0.6 | 320 | 3.0 | 8000 | 32.0 | 20.7 | 13.0 | − | 15.9 | 78.5 | [15] |
| 9 | Na/Fe3O4 | 300 | 3.0 | 2500 | 30.6 | 4.1 | 15.1 | 38.3 | 42.88 | − | [16] |
| 10 | 2K20Fe5Rh-SiO2 | 250 | 5.0 | 7000 | 18.4 | − | 46 | 15.9 | 15.9d | 21.4c | [17] |
| 11 | In2Fe/K-Al2O3 | 300 | 2.0 | 4000 | 36.7 | 7.4 | 14.6 | − | 42 | − | [18] |
| 12 | 0.6S-KCFZ | 320 | 5.0 | 3000 | 36.1 | 12.1 | − | − | 20.2 | 50.7 | [19] |
| 13 | 0.6SKCFZ/CuZnAlZr (1:1) | 320 | 5.0 | 12000 | 36.6 | 22.3 | − | − | 18.2 | 173.9 | [19] |
| 14 | 4.7KCFZ/CuZnAlZr (1:1) | 300 | 5.0 | 3000 | 27.0 | 25.4 | − | − | 24.6 | 42.0 | [20] |
| 15 | 10Mn1K-FeC | 300 | 3.0 | 6000 | 40.5 | 33.4 | − | 7.0 | 10.5 | [21] | |
| 16 | KFeCu/a-ZrO2 | 320 | 4.0 | 12000 | 25.7 | − | − | 26.1 | 125.0 | [22] | |
| 17 | Na-ZnFe@C (10% CO co-feeding) |
320 | 5.0 | 9000 | 32.8 | 89.2* | − | 29.5 | 29.5 | 366.6 | [23] |
| 18 | Na-ZnFe@C | 320 | 5.0 | 9000 | 38.4 | 7.6 | 15 | 20.3 | 20.3 | 158.1 | [23] |
| 19 | Cr(1%)-CuFe | 320 | 4.0 | 6000 | 38.4 | 14.8 | 17 | 22.1 | 29.2 | 104.1 | [24] |
| 20 | Cr(1%)-CuFe | 320 | 4.0 | 24000 | 30.1 | 17.5 | 22 | 16.1 | 20.4 | 217.3 | [24] |
| 21 | Cr(1%)-CuFe | 320 | 4.0 | 48000 | 24.0 | 23.3 | 21 | 13.8 | 17.5 | 268.5 | [24] |
| 22 | MnCuK-FeC/ CuZnAlZr |
300 | 3.0 | 6000 | 42.1 | − | − | 9.3 | 15.5 | [25] | |
| 23 | sp-CuNaFe | 310 | 3.0 | 28800 | 32.3 | ~17 | ~34 | − | ~10 | 153 | [26] |
| 24 | K/Cu-Zn-Fe | 300 | 7 | 500e | 44.2 | 5.9 | − | − | 19.5 | 110.6f | [27] |
| 25 | RhFeLi/TiO2 | 250 | 3 | 6000e | 15.7 | 12.5 | − | − | 35.8 | 60.6f | [28] |
| 26 | Cu25Fe22Co3K3-CuZn1.0K0.15 | 350 | 6 | 5000e | 32.4 | 45.3 | 12.9 | − | ~6.53 | <27.3f | [29] |
| 27 | RhFe/SiO2 | 260 | 5 | 6000 | 23.7 | 23.7 | 30.9 | 16.4 | 16.4 | ~59.9 | [30] |
| 28 | 90Fe10Co(1.0)K | 240 | 1.2 | 1500 | 14.5 | 45.5 | − | 5.9 | 5.9 | ~3.3 | [31] |
| *CO conversion; a: g/(mL·h); b: mmoL/(g·h); c: mL/(g·h); d: EtOH; e: mL/(mL·h); f: g/(L·h). | |||||||||||
![在 NaFe@C−CZA 多功能催化剂上将CO2加氢转化为C2+醇的可能反应途径(a)和邻近效应(b)[12] ,(c)用于C2+醇合成的CO/CO2共进气金属-分子筛级联催化工艺[](/data/tmp/figure/3dfe7161f9a4064c0625739a3e2d3b7b.jpg)
![CuFe(a)、(c)和(e)和Cr(1%)-CuFe(b)、(d)和(f)催化剂上的CO2加氢原位DRIFTS[24]](/data/tmp/figure/99ecf9e542f1777b0054de16ea02a037.jpg)
![还原后(a)、(c)和反应后(b)、(d)KFe、KFe/S1和KFe/Z5-36催化剂的XRD和XPS谱图[39]](/data/tmp/figure/acb7e7f087e1815e8dbfec7a71c830fc.jpg)
![CO2加氢合成乙醇的碳层电子缓冲效应示意图[23]](/data/tmp/figure/4e38f224a03096d3d8c0cfeea1774ca8.jpg)
![催化剂的(a)Cu 2p XPS光谱,(b)Cu LM2 XPS光谱和(c)Fe 2p XPS光谱谱图[26]](/data/tmp/figure/ccae75375c1cd817200cd586fa1b4516.jpg)
![样品的(a)Zr 3d XPS谱图和(b)O 1s XPS谱图,以及在(c)CO预吸附TPSR-MS谱图中m/q =16和28的MS信号;(d)KFeCu/a-ZrO2被NH3毒化过程示意图[22]](/data/tmp/figure/84b11a79adb2a71582e4e3d4d1485a87.jpg)
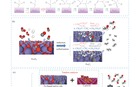
Table3
不同工况下放电参数
| 反应模式 | Upp/kV | Ccell/pF | Cdiel/pF | Ub/kV | E:N/Td |
| 仅等离子体 | 23.34 | 20.33 | 82.02 | 2.08 | 80.34 |
| 等离子体+10 wt.% Cu/γ-Al2O3 |
23.46 | 30.65 | 82.62 | 2.20 | 96.62 |
| 注:Upp为峰峰值施加电压;Ccell为电池电容;Cdiel为介电电容,本文中为石英管;Ub为击穿电压;E:N为电场强度和粒子数密度的 比值。 |
|||||
Table2
动力学模型参数
| 参数 | 数值 |
| 截止时间/s | 3.5、2.6、1.5 |
| 停留时间/s | |
| 气体温度/K | 423.15 |
| 初始电子温度/eV | 3 |
| 压力/MPa | 0.1 |
| 体积/cm3 | 6.97 |
| 功率沉积/(W·cm−3) | 2.15×105 |
| 脉宽/ns | 100 |
| 脉冲间隔/s | |
| 初始气体体积分数 | CO2:0.25 H2:0.75 |
Table1
NTP强化Cu催化CO2加氢反应机理模型中包含的E-R反应
| 反应式序号 | 反应 | 黏附系数/γ | 参考文献 |
| S6 | CH2O(S) + H → CH3O(S) | 10−4 | [23] |
| S7 | CH3O(S) + H → CH3OH(S) | 10−4 | [22] |
| S8 | CH2O + H(S) → CH3O(S) | 10−4 | [23] |
| S9 | CH3O + H(S) → CH3OH(S) | 10−4 | [23] |
| S10 | H(S) + OH → H2O + Cu(S) | 10−5 | [23] |
| S11 | H + OH(S) → H2O + Cu(S) | 10−4 | [23] |
| S12 | H + O(S) → OH(S) | 10−4 | [23] |
| S13 | H(S) + O → OH(S) | 10−5 | [23] |
| S14 | CO + H(S) → HCO(S) | 8×10−6 | [22] |
| S15 | CO(S) + H → HCO(S) | 10−4 | [22] |
| S16 | HCOO(S) + H → HCOOH(S) | 10−6 | [22] |
| S17 | CO2(S) + H → HCOO(S) | 10−6 | [22] |
| S18 | CO2(S) + H → COOH(S) | 6×10−4 | [22] |




主办单位:煤炭科学研究总院有限公司 中国煤炭学会学术期刊工作委员会